Spray Analyses in Pharmaceutical & Medical Devices
Inhaled Drug Delivery Device Testing
Imaging Contract Services Laboratory
Oxford Lasers’ imaging laboratory provides access to the latest advanced imaging techniques to analyse the dynamic performance of inhalers. Pharmaceutical and medical device companies are focusing on increasing sustainability with new low-carbon propellants and alternative designs. Oxford Lasers supports these sectors with a full array of testing services available including:
- Dynamic plume geometry
- Spray pattern
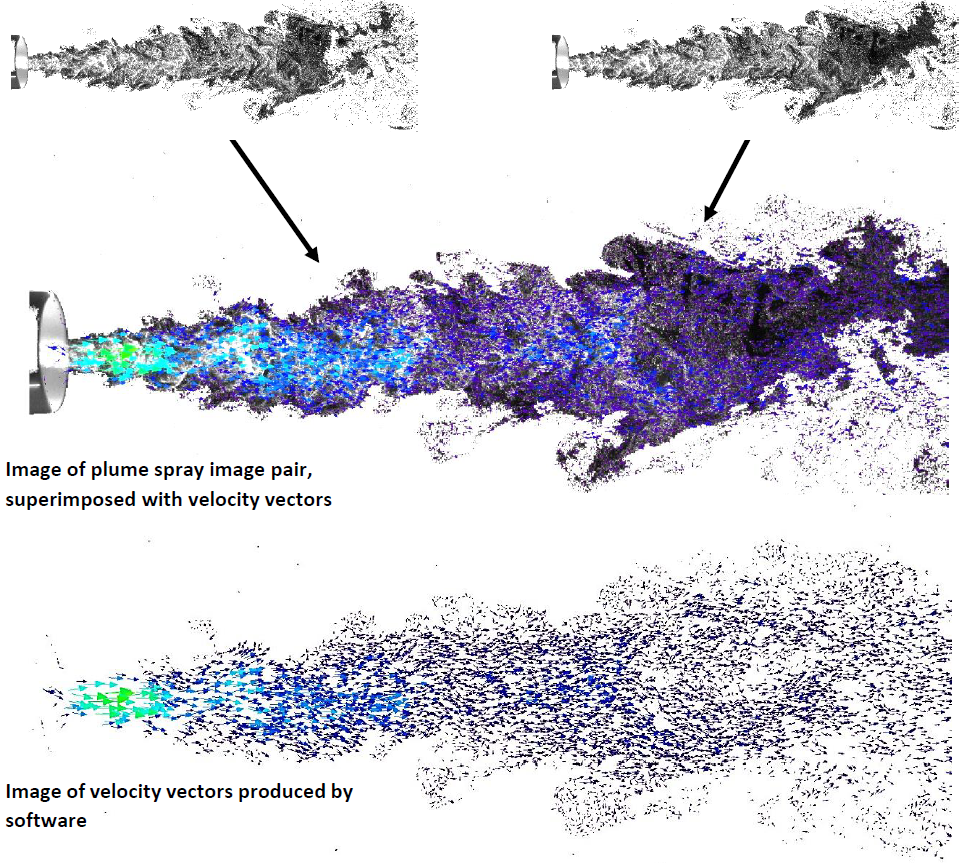
- Flow field velocity
- Actuation stability and consistency data
- and more…

Development Testing Partner
Advanced imaging analysis enables manufacturers to adjust formulations, evaluate designs and determine effectiveness for delivery of therapeutic products. Whether you are working on a:
- SMI (softmist inhaler)
- pMDI (pressurised metered dose inhaler)
- BAI (breath actuated inhaler)
- DPI (dry powder inhaler)
- intra-nasal device
- any latest smart device
Oxford Lasers is able to provide rapid screening tests to bring your product to market faster.
Evaluate Device Performance and Consistency
Pharmaceutical and medical device companies can gain insight into their product delivery by evaluating plume geometry, spray velocity, fluidisation and impact with advanced imaging techniques at Oxford Lasers. We help our customers answer the following questions:
Q: How do you know if your actuation events are consistent between events or between devices?
A: Oxford Lasers provides advanced imaging services to capture and evaluate plume development, spray pattern, velocity and full release profiles of inhaler performance. We enable customers to understand if the device is behaving in a consistent manner by fully investigating the spray behaviour.
Q: How would you know if a change in propellant impacted your formulation?
A: Oxford Lasers provides comparative dynamic plume evaluation for understanding if a new product meets performance and equivalency requirements by imaging plume development, and conducting velocity analysis to create comparative dose release profiles.
Q: How do you know if your powder formulation flows through your device and releases its API effectively?
A: Oxford Lasers enables our customers to evaluate powder bed break-up, product release and impact of API through high-speed imaging. We provide imaging analysis to evaluate break-up, fluidisation, spray pattern and plume geometry in the evaluation of dry powders.

Contract Services
Oxford Lasers has over 20 years of experience supporting global companies with advanced imaging techniques. Providing analytical data to support the qualification and validation of spray devices, we are a UKAS-accredited laboratory ISO17025:2017 (accreditation no. 20625) to test pharmaceutical spray devices with high-speed image capture of spray plume (plume geometry and spray pattern).



